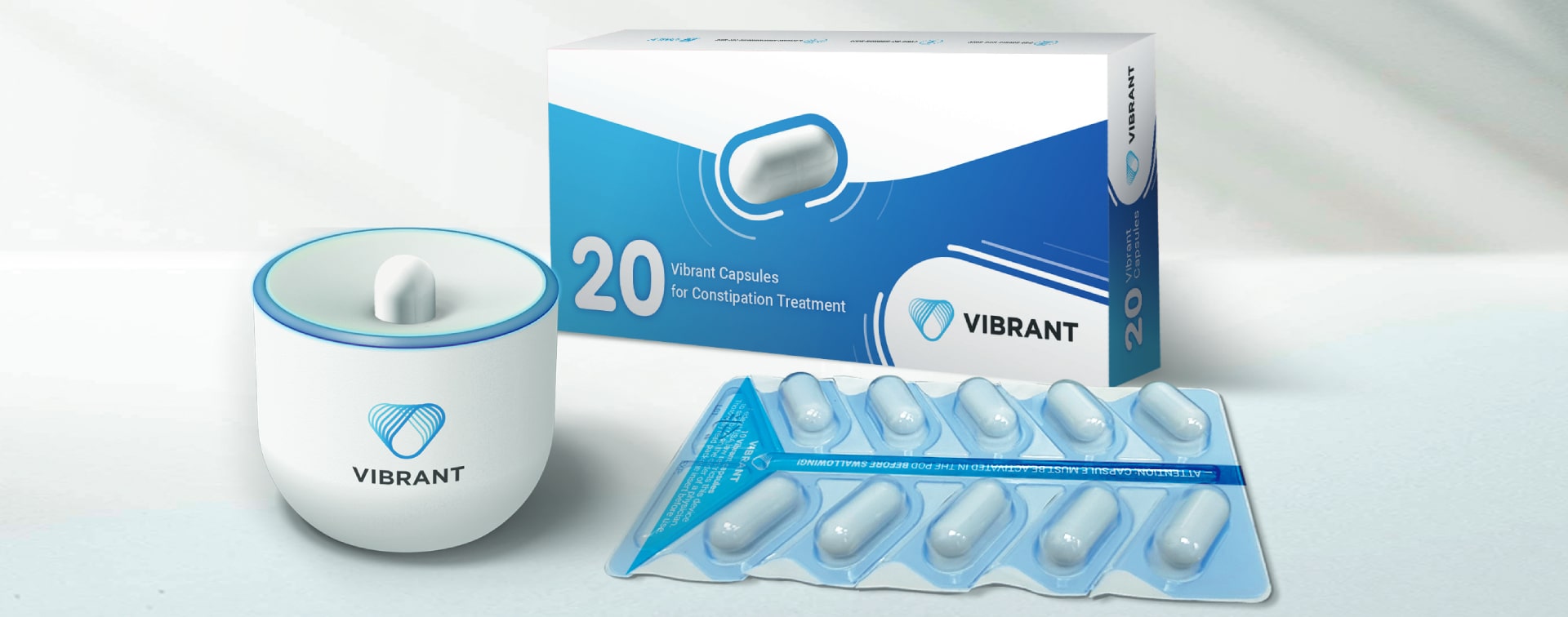
Vibrant reports positive efficacy and safety in a pivotal study for its namesake Vibrant System
Vibrant, announced that the Vibrant System for chronic idiopathic constipation met its primary safety and efficacy endpoints in the V-270 pivotal trial. Based on the study’s positive findings, CEO Lior Ben Tsur announced the company has submitted a de novo classification request to obtain marketing clearance for the Vibrant System from the U.S. Food and Drug Administration (FDA).
The Phase III trial evaluated the safety and efficacy of the Vibrant System in 312 patients at over 70 clinical centers across the United States, including Harvard Medical School, Augusta University, Georgia’s Health Sciences University, University of Michigan, and Houston Methodist Hospital. “The development of the Vibrant System highlights the kind of scientific approach we need to address so many ailments today,” said gastroenterologist Satish Rao, M.D., Ph.D., a professor of medicine at the Medical College of Georgia, and a principal investigator (PI) in the phase III study. “The goal of Vibrant is to address a real problem in chronic constipation, which has made life very challenging for those who suffer from it. We believe the Vibrant System is a meaningful step towards achieving that goal.”
The new Vibrant’s non-drug system provides new hope to the millions of Americans who suffer from chronic idiopathic constipation without satisfactory relief from Laxatives. Current studies show that 62%-78% of CIC patients taking OTC medication may experience little or no satisfaction with the medication’s effect on their constipation and CIC-specific abdominal symptoms2.
Vibrant’s non-drug system leverages research exploring the Gut-Brain axis—the connection between the body’s gastrointestinal tract and enteric nervous system. Vibrant brings a new approach to the problem. Augmenting the circadian rhythm, the body’s natural biological clock, via the gut-brain connection, allows the bowel function to return to activity naturally, providing significant constipation relief.
“Vibrant’s novel treatment improves patients’ constipation symptoms while offering health care providers a new tool to assist theirs with chronic constipation patients.” said CEO Lior Ben-Tsur. “We look forward to working with the FDA through the review process to make this treatment available to clinicians and patients.”
About Vibrant
Vibrant is the pioneer of the Synchronized Activation Method™, a novel technology system relying on the science of the gut-brain connection via the Enteric Nervous System (ENS) pathway.
Vibrant is a proprietary, biocompatible treatment for chronic idiopathic constipation (CIC). The treatment is composed of a drug-free, orally administered capsule that induces bowel movements. The capsule is directed by a pod, which activates and sends the operating instructions to the capsule.
The Vibrant capsule is an investigational device limited to investigational use in the United States. It is currently under FDA review and is not available for sale.
To learn more about Vibrant, visit www.vibrantgastro.com
1. Market Insights, Epidemiology & Market Forecast”2030Market Insights, Epidemiology & Market Forecast”2030
2. Lacy B et al., Lessons Learned: Chronic Idiopathic Constipation Patient Experiences with Over-the-Counter Medications, PLOS ONE 16(1):e0243318.